Agilent Technologies - EZChrom Elite
Stand-alone and Client/Server Chromatography Data System
Users in regulated environments can take advantage of a comprehensive array of features for GLP/GMP concerns. Complete audit trails and system logs to document and record all changes to the system, highly flexible user security for access and operational privileges, and electronic signatures and signoffs are just some of the special features in EZChrom Elite.
Not all labs have the same GLP related requirements. With EZChrom Elite, the lab administrator can enable and configure the GLP related features of the software to match the needs and SOPs of the lab.
Active Questions & AnswersAsk a Question
Need Equipment Support?
Documents & ManualsView All Documents
Features of EZChrom Elite
Multi-User Logins and Security EZChrom Elite provides an comprehensive set of rights and permissions that can be assigned to groups or individual users. Security is integrated with Windows Active Directory domain lists, so that lab managers can easily control what software operations are granted to a user, what instruments can be controlled, and which project folders can be accessed. The flexibility of the security subsystem lets each laboratory tailor EZChrom Elite to fit their own individual workflows and SOPs.
Audit Trails From a fundamental need to unambiguously track changes to important files (who, what, when, why) to automatically monitoring changes to methods, data, sequences and report templates, EZChrom Elite's audit trails are fully automated and completely detailed to provide assurance that you have a total history record of all events. Lab administrators can enable audit trails on a file-by-file, project-by-project or entire enterprise basis.
Activity Logs EZChrom Elite provides several logs that track system activities. Instrument activity logs automatically record user access and log off, the start and end of single injections and sequences, as well as instrument errors and warnings. A system activity log automatically records all user administration operations. All activity logs can be printed, exported and archived, so that you can manage, as well as access, important software records.
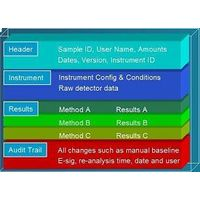
Result Traceability Knowing the precise instrument settings and data handling parameters (calibration curves, integration conditions, timed events, etc.) that were used to generate a result are critically important in many laboratories. EZChrom Elite's unique compound data file architecture automatically embeds the acquisition and analysis method parameters in the same file as the data and results. The same data file also includes instrument configuration and status information, audit trails and more. Regardless of the number of times you modify parameters and reanalyze your data, the exact method used to obtain a result is always available. The embedded method can be recalled, viewed, even re-used in further data acquisition or re-analysis if desired. This unambiguous traceability between method conditions and result provides users with full assurance that all results can be unambiguously associated with the actual acquisition conditions.
21 CFR Part 11 Few initiatives have had a greater impact on the regulated laboratory than 21 CFR Part 11. Since its release several years ago, more and more laboratories and enterprises have adopted the 21 CFR rules as requirements for their lab processes. EZChrom Elite's built-in GLP feature set thoroughly addresses the 21 CFR Part 11 rules, so that when implemented in the laboratory and used in conjunction with proper internal practices, they enable the laboratory to fully comply with 21 CFR Part 11 in the operation and handling of all chromatography information.
General Specifications
There are no General Specifications available.